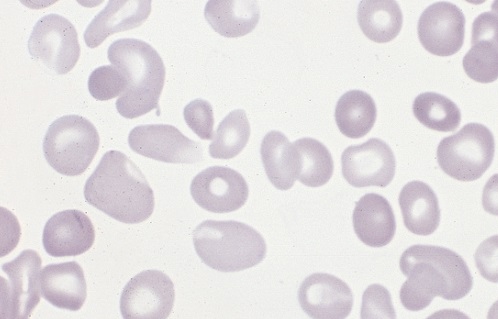
Polycythemia occurs when bone marrow is manufacturing too many red blood cells, thereby increasing the volume percentage of red blood cells in the blood. It can also be caused by a low plasma count in relation to blood cells, causing an imbalance. These excess cells, in turn, thicken the blood, which slows down the overall blood flow leading to more serious issues, such as blood clots. Polycythemia is essentially the opposite of anemia, which is when there are too few blood cells being produced.
Neonatal is a term used to describe babies within the first 28 days of life. Neonatal polycythemia is diagnosed when a baby’s blood is composed of more than 65% red blood cells. This is a common problem with newborns, but that number is expected to decrease within a few hours if everything is normal.
Some babies who are at a higher risk include those who are born small for their gestational age, babies who are post-term, infants of mothers with diabetes, twin-to-twin blood transfer in utero, low oxygen levels in fetal blood, and babies born with chromosomal abnormalities.
It can be hard to diagnose this condition when babies are first born, because, aside from a high blood viscosity, babies might be asymptomatic before showing any metabolic changes due to this disorder. Samples should be taken from a largely free-flowing blood vessel in order to get an accurate hematocrit result. Signs that blood is affected include poor blood flow returning to a site after pressure is applied (peripheral perfusion), and blood having a ruddy, dusky appearance. There are also other clinical symptoms that include lethargy, irritability, tremors, seizures, lack of interest in feeding, hypoglycemia, rapid breathing (tachypnea), and bluish/grayish skin coloration (cyanosis).
There are several other conditions that share similar symptoms with neonatal polycythemia, which is why it is vital to rule them out before attempting any course of treatment. Some conditions that are similar include hypoglycemia, neological dysfunction, renal failure, or respiratory issues. Once a firm diagnosis is confirmed, there is a recommended course of action.
Not all babies require treatment, but if there are signs of metabolic distress, the first priority is to lower the hematocrit by performing a partial exchange transfusion (PET). Either a saline solution may be used or a 5% protein solution. Saline is the preferred material because it won’t risk infection and has a better price point. It is strongly advised to avoid fresh frozen plasma because studies have shown a correlation between its use and necrotizing enterocolitis.